
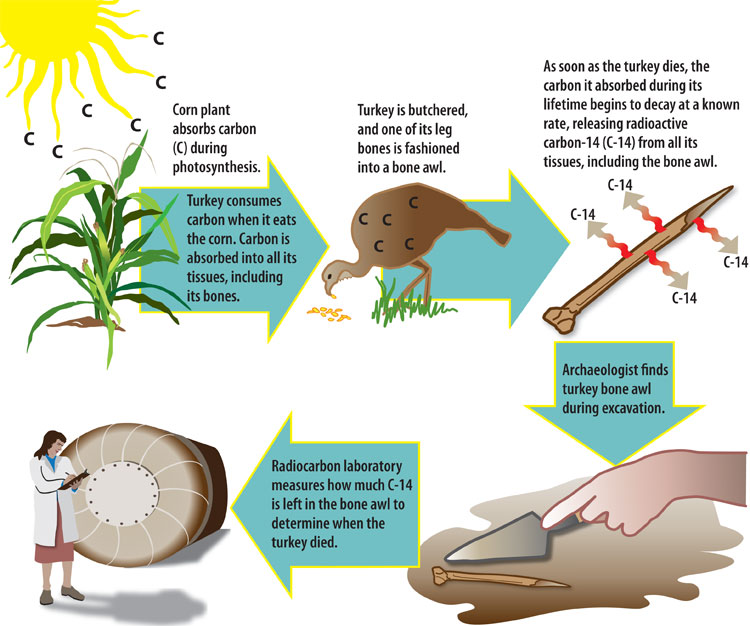
Radiocarbon ( 14C) produced in the atmosphere by the decay of nitrogen ( 14N) enters the living organisms through the biological carbon cycle. Some other methods of radioactive dating.Uptake of 14CO2 by plants through photosynthesis But it’s the most accurate dating tool at archaeologists’ disposal, thanks to carbon-14’s predictable disappearing act. Radiocarbon dating isn’t a silver bullet: Context is everything, and it can be hard to determine if there’s a temporal relationship between two objects at an archaeological site. ( See how radiocarbon dating helped researchers determine when this ship sank.) Modern statistical methods and updated databases allow scientists to take humans’ effects on Earth’s atmosphere into account. Nuclear testing affects radiocarbon levels, too, and dramatically increased carbon-14 levels starting in the 1950s. With the dawn of the Industrial Age, humans began emitting much more carbon dioxide, diluting the amount of radiocarbon in the atmosphere. Over 60,000 years old, and they can’t be dated at all.Ĭalibration presents another challenge. Age is also a problem: Samples that are older than about 40,000 years are extremely difficult to date due to tiny levels of carbon-14. Inorganic materials can’t be dated using radiocarbon analysis, and the method can be prohibitively expensive. The method has limitations: Samples can be contaminated by other carbon-containing materials, like the soil that surrounds some bones or labels that contain animal-based glue. Scientists are turning to radiocarbon analysis to monitor when ivory was poached. Since Libby’s discovery, radiocarbon dating has become an invaluable tool for archaeologists, paleontologists, and others looking for reliable dates for organic matter. He won the 1960 Nobel Prize in Chemistry for coming up with the method. ( Discover other archaeological methods used to date sites.)Ĭhemist Willard Libby first realized that carbon-14 could act like a clock in the 1940s. The result is then calibrated and presented along with a margin of error.

To date an object, researchers use mass spectrometers or other instruments to determine the ratio of carbon-14 and carbon-12. But the amount of carbon-14 in tree rings with known ages can help scientists correct for those fluctuations. There’s a catch: Atmospheric carbon fluctuates over time. And since animals and plants stop absorbing carbon-14 when they begin to decay, the radioactivity of the carbon-14 that’s left behind reveals their age. The less radioactivity a carbon-14 isotope emits, the older it is. Since carbon-12 doesn’t decay, it’s a good benchmark against which to measure carbon-14’s inevitable demise. That half-life is critical to radiocarbon dating. Every 5,730 years, the radioactivity of carbon-14 decays by half. On the other hand, carbon-14 is radioactive and decays into nitrogen-14 over time. The most abundant, carbon-12, remains stable in the atmosphere. Humans and other animals ingest the carbon through plant-based foods or by eating other animals that eat plants. While plants are alive, they take in carbon through photosynthesis. And with the help of radiocarbon dating, researchers can use that decay as a kind of clock that allows them to peer into the past and determine absolute dates for everything from wood to food, pollen, poop, and even dead animals and humans. Over time, carbon-14 decays in predictable ways. Nothing good can last-and in the case of carbon-14, a radioactive isotope found in Earth’s atmosphere, that’s great news for archaeologists.


 0 kommentar(er)
0 kommentar(er)
